Abstract
Background: Sickle cell disease (SCD) is an inherited systemic disorder in which sickle hemoglobin (HbS) polymerization triggers red blood cell sickling, chronic hemolytic anemia, and recurrent episodes of vaso-occlusion. SCD-related complications lead to acute and chronic life-threatening events, cumulative organ damage, disability, and early mortality. Voxelotor (Oxbryta ®) tablets are approved in the United States for treatment of SCD in adults and adolescents aged ≥12 years, based on the efficacy and safety data from the randomized, placebo-controlled, multicenter HOPE trial. Voxelotor is an oral, once-daily HbS-polymerization inhibitor that has been shown to increase hemoglobin (Hb) levels and reduce markers of hemolysis. The Retrospective Study to Evaluate Outcomes in Patients with Sickle Cell Disease Treated with Oxbryta (RETRO) is designed to collect, aggregate, and characterize real-world, retrospective laboratory and clinical data on adults and adolescents with SCD treated with voxelotor as part of their usual care at multiple clinical centers in the United States.
Methods: RETRO is a multicenter, post-marketing, retrospective study of approximately 300 patients (aged ≥12 years) with SCD from 10 US study sites. Independent SCD expertise is provided by a steering committee to inform the design and conduct of the voxelotor registry. Clinical and laboratory data have been collected and aggregated 12 months before initiation of voxelotor treatment and compared with post-treatment data outcomes. Patients with documented SCD (all genotypes) who received voxelotor treatment for ≥2 consecutive weeks were included in this analysis. Only data available from patients' medical records (and other secondary data sources) 1 year before and up to 1 year after the first voxelotor dose were documented in de-identified case report forms via an electronic data capture system.
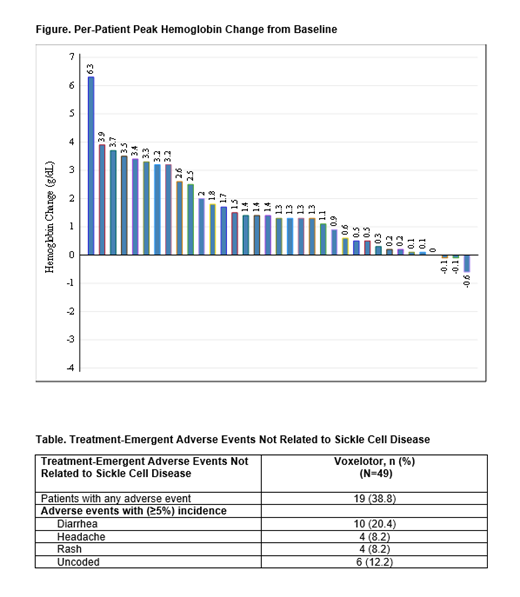
Results: Forty-nine patients whose data were entered at 5 sites at the time of data cutoff (June 25, 2021) were included (mean age [SD]: 34.3 [12.91] years; 57.1% female; 85.7% HbSS and 6.1% HbSβ 0 genotype). Mean (SD) duration of voxelotor treatment was 48.1 (23.0) weeks. The initial prescribed voxelotor dose strengths (n, %) were 500 mg (4, 8.2%), 1000 mg (7, 14.3%), and 1500 mg (38, 77.6%). Rationale for prescription (n, %) included reduction of anemia (36, 73.5%), reduction in frequency of vaso-occlusive crises (23, 46.9%), reduction in pain (34, 69.4%), reduction in the need for blood transfusion (8, 16.3%) and other (5, 10.2%); more than 1 reason may have been selected. In 35 patients with recorded baseline and post-treatment Hb values, the peak observed post-treatment Hb (mean [SD]) was 9.4 (2.44) g/dL, an increase of 1.6 (1.5) g/dL from baseline (7.8 [2.02] g/dL). Fifty percent (11/22) of patients had a clinical response (Hb increase of >1.0 g/dL from baseline) within 12 months of voxelotor treatment. Per-patient peak changes in Hb during the study period showed that 62.9% of patients experienced a response at some time up to 12 months during treatment (Figure). Change in hemolytic markers was also evaluated. In patients with recorded baseline and post-treatment reticulocyte percentage (N=19) and indirect bilirubin (N=24), the mean (SD) absolute post-treatment value was 7.4% (4.65%) for reticulocyte percentage, a decrease of 4.9% (6.63%) compared with baseline (12.4% [8.32%]), and 1.9 (1.66) mg/dL for indirect bilirubin, a decrease of 17.7 (81.83) mg/dL compared with baseline (19.6 [81.82] mg/dL). The most common non-SCD-related treatment-emergent adverse events (AEs) were diarrhea, headache, and rash (Table); 19 (38.8%) patients reported ≥1 AE, and most non-SCD-related AEs were mild in severity.
Conclusions:RETRO is the first multicenter, retrospective study to examine the real-world effectiveness of voxelotor and describe the observed changes in laboratory and clinical outcomes after ≥2 weeks of therapy. This study shows that voxelotor treatment was associated with increased Hb levels and decreased hemolytic markers. The safety data are consistent with those from the HOPE trial. Further evaluation is needed, with additional data from all 10 sites, and will be presented later.
Funding: This study was supported by Global Blood Therapeutics.
Achebe: Fulcrum Therapeutics: Consultancy; Pharmacosmos: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees. Clay: GBT: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria. Nero: Global Blood Therapeutics: Consultancy; Editas Medicine: Consultancy; bluebird bio: Consultancy; Novartis: Consultancy. Osunkwo: Terumo: Consultancy; Global Blood Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Acceleron: Consultancy; Forma Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Health and Services Administration: Research Funding; Patient Centered Outcomes Research Instituted: Research Funding; Micella Biopharma: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Chiesi: Consultancy; Cyclerion: Consultancy; Emmaus: Consultancy. Idowu: Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Ironwood: Research Funding; Forma Therapeutics, Inc.: Research Funding; Pfizer: Research Funding. Shah: Novartis: Research Funding, Speakers Bureau; Alexion: Speakers Bureau; Emmaus: Consultancy; GBT: Consultancy, Research Funding, Speakers Bureau; Guidepoint Global: Consultancy; CSL Behring: Consultancy; GLG: Consultancy; Bluebird Bio: Consultancy. Curtis: GBT: Consultancy. Minniti: Bluebird Bio: Other: Endpoint adjudicator ; F. Hoffmann-La Roche Ltd: Consultancy; Chiesi: Consultancy; Novo Nordisk: Consultancy; Forma: Consultancy; Novartis: Consultancy; GBT: Consultancy; CSL Behring: Other: Endpoint adjudicator .

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal